Summary: (PINK:PRED)
- Two days ago, Predictive announced that it was partnering with a little-known Chinese manufacturer called Jiangsu Dablood Pharmaceutical Co. Ltd. (“Dablood”) to manufacture and distribute rapid tests capable of detecting COVID-19 in as little as 15-minutes.
- Contrary to Predictive’s press release, which calls its partner “one of very few companies approved by the Chinese government,” we found that Dablood is NOT an approved manufacturer of COVID-19 tests in China.
- Instead, multiple investigative reporters in China have exposed that Dablood’s COVID-19 tests have been sold in China without medical or production licenses, which is illegal. One reporter specifically mentioned that the conduct was suspected to be fraudulent.
- Predictive’s CEO, Brad Robinson, has previously been sued over claims of non-compliant marketing of a medical product and claims of falsely stating that his product would be pitched by Dr. Oz and the Gates Foundation. Predictive’s former Chairman had been charged by the SEC over allegations of issuing false press releases.
- We think this COVID-19 rapid testing stunt is the company’s last attempt at salvaging itself, as Predictive’s financials suggest to us that the company is months, if not weeks, away from insolvency.
- We see 100% near-term downside in shares of Predictive and expect that Predictive must continue to dilute equity through near-term stock sales in order to survive.
Initial Disclosure: After extensive research, we have taken a short position in shares of PRED. This report represents our opinion, and we encourage every reader to do their own due diligence. Please see our full disclaimer at the bottom of the report.
Update 3/31/2020: After identifying that Dablood is NOT approved to offer its tests domestically in China, Predictive has now stated that Dablood is a partner entity of Darui Biotechnology, which is a subsidiary of Da An Gene Co, an entity that is on the approved list. Predictive then stated “As such, Dablood operates under the Da An Gene Co., Ltd. license.”
This once again appears to be false, as Dablood announced in a letter dated the 16th that it was planning to apply for domestic approval for its COVID-19 test kits (and thus has not been approved.)
Predictive’s Rapid COVID-19 Test Announcement: A Pink-Sheet Company Claims to Have Landed a Game-Changing Deal
Two days ago, Predictive Technology Group (PINK:PRED) announced that it intends to immediately begin importation and distribution of a rapid 15-minute COVID-19 test branded Assurance AB.
Such an offering would be major for Predictive. The two largest lab corporations in the nation, LabCorp and Quest Diagnostics, are currently offering tests that return results in 3-5 days. An accurate 15-minute test for COVID would be a game changing development for detection and treatment of the virus that has paralyzed the world. A quick test that can be mass produced is one of the key problems being worked on collectively, around the world, to help wrangle in the COVID-19 pandemic [1,2,3].
It would also be an unexpected development. Predictive is a small, struggling provider of stem cell products to various stem cell clinics nationwide. A switch to mass-provider of COVID-19 tests is a major and sudden business pivot.
Nonetheless, the stock ripped on the press release announcing the test, more than doubling on 15x normal volume. While the announcement was a surprise bit of “news” for followers of the company, we found it to be completely expected.
Predictive has a long, storied history of questionable deals and announcements, as we thoroughly covered in a detailed exposé last July. Among these:
- Predictive’s former long-serving Chairman/director and key backers had been alleged by the SEC and state regulators to have engaged practices ranging from issuing false press releases to boiler-room-fueled pump and dumps.
- Predictive’s CEO, Brad Robinson, has been sued over allegations of “non-compliant marketing of a medical product”. Another complaint alleged that Robinson made grandiose claims about a medical product’s efficacy and that it would be pitched by Dr. Oz, the Gates Foundation, and others. None of the claims ever materialized.
Beyond history, the company looks to be bordering on insolvency, providing a desperate backdrop for what we believe is nothing more than a PR stunt involving these COVID-19 tests.
As of the latest quarter-end, Predictive had only $255,502 (figure not in thousands) of cash compared to current liabilities of almost $10 million. [Pg. 2] It had burned $9.9 million of operating cash in the latest 6-month period and reported a net loss of $33.9 million during the same time frame.
The company appears to be on life support. To survive, it has been relying on private loans. Per the latest financials, these loans were recently converted into 12,947,833 shares of common stock. [Pg. 40]
With the COVID-19 outbreak, we suspect the company’s sales of stem cells, which accounted for 99% of its revenue, have further nose-dived. [Pg. 10]
Without stock sales, we think the company would be defunct in a matter of months, if not weeks. And what better way to sell stock than with the announcement of a game-changing new COVID-19 test right during the height of a global pandemic?
Unfortunately, the company’s announcement of a new coronavirus test fails to stand up to even basic scrutiny. We think the announcement essentially amounts to a sham and we call on the company to answer the questions that we e-mailed investor relations days ago – and to provide immediate clarification to the market on its supposed COVID-19 test.
Predictive’s Claims About Its COVID-19 Test Don’t Stand Up to Basic Scrutiny
We went through the company’s announcement point-by-point and have identified glaring irregularities and what appear to be outright falsehoods.
1. Claim: “To the best of Predictive’s knowledge, Dablood Pharmaceutical is one of very few companies approved by the Chinese government to co-develop and manufacture rapid antibody tests”.
Reality: Dablood is NOT one of the companies approved by the Chinese government to offer COVID-19 tests. The official list of approved COVID-19 test providers is published by the Chinese government, was updated as of March 17th, and does not include Dablood or its affiliates. (Update 3/31: Predictive now claims that Dablood is partnered with a company that is owned by a subsidiary of an entity that is approved. Per our update above, this still does NOT mean that Dablood or its test kit is approved.)
The use of the language “to the best of Predictive’s knowledge” in relation to the approval strikes us as disingenuous. This was an easily confirmable fact that took us minutes to find.
Dablood was issued a permit in November 2019 from the Jiangsu provincial government to develop “reagents for enzyme detection” but is NOT licensed for COVID-19 testing.
Additionally, various reports [1,2] in Chinese media suggested that Dablood’s test was in line to be fast tracked for approval back in February. But, to our knowledge, no such approval has taken place.
On the contrary, multiple investigative reporters in China have exposed that the Dablood COVID-19 test kits have been sold without licensing or permitting, a practice that is illegal in China. (1, 2)
On March 16th, an investigative reporter found that the 15-minute at-home COVID-19 test kits from Dablood (i.e. “Darbo Pharmaceutical”) had been sold without a proper medical device registration certificate.

Several days later on March 19th, another investigative reporter corroborated those findings, and confirmed that the Dablood tests were being sold through various online merchants without a medical device registration certificate and without a production license number. The reporter made it clear that those irregularities constituted clear signs of fraud.
We called Dablood during working hours at the number on its contact page to ask about the status of its approval, but the line gave a busy signal each time (we called about 8 times). We also emailed the company but have not heard back as of this writing.
2. Claim: “The Company cautions that if the federal government decides to restrict adequate reimbursement or the foreign import of products manufactured in Asia, the Company will not be able to provide access to these tests in the U.S.”
Reality: This disclosure was the last line of Predictive’s press release. What it means (and what retail investors buying into the stock seem to have ignored) is that Predictive has no specific approval from requisite U.S. authorities to provide this test.
As of today, the FDA has issued 17 Emergency Use Authorizations to companies offering COVID-19 tests, and Predictive isn’t one of them. The FDA has not yet approved any foreign provider of COVID tests as of March 22nd, which obviously includes Dablood.
3. Claim: “Dablood Pharmaceutical is one of the largest diagnostic kit manufacturers in China and is currently producing up to 1.5 million units of the rapid antibody test per day in China.”
Reality: Dablood (Chinese name: 江苏达伯药业有限公司) is a relatively young company. Chinese registration data shows that the company was incorporated in 2017 and has fewer than 50 employees.
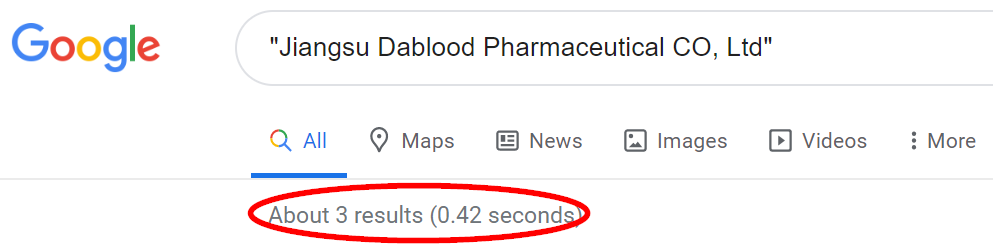
A basic Google search of the company’s English name shows a grand total of 3 results, all relating to the Predictive press release. We believe this is hardly a sign that the company has a sprawling U.S. (or even international) presence.
As to the claim of “currently producing” 1.5 million tests per a day, a February 6th Chinese-language article on Dablood’s coronavirus test operations stated that “The production line was upgraded on January 31, with an average daily output of 20,000 units.”
Going from 20,000 to 1.5 million in a month strikes us tremendously unlikely, especially since the test apparently hasn’t been approved domestically and the company only announced plans to launch its test internationally 3 days ago.
4. Claim: “Dablood Pharmaceutical has been recognized by the Chinese government for its effort of developing and producing testing products to help the detect the COVID-19 and has received approval for distribution of its test across the domestic Chinese market”
Reality: This claim appears to be outright false, per the official Chinese government list of approved COVID-19 test providers.
5. Claim: Brad Robinson, CEO of Predictive, was quoted in the company’s press release as saying: “Domestic and international governmental agencies, healthcare groups, pharmacies and employers have shown a strong interest in our test.”
Reality: We had a hard time believing that international agencies, healthcare groups, pharmacies and employers had already shown a “strong interest” in the COVID-19 test that Predictive first announced that same morning.
We emailed Predictive’s investor relations and asked them to substantiate that claim and name a single agency, healthcare group, pharmacy or employer that had shown interest in the test and to detail what that interest consisted of.
Predictive’s response so far: Silence
6. Claim: “Company has notified the U.S. Food and Drug Administration of its intent to immediately distribute the validated [COVID-19] test to laboratories and healthcare workers at the point-of-care in the U.S.”
Reality: Virtually all of the company’s revenue to date has been derived from selling stem cell products manufactured in its Utah lab. [Pg. 8] Predictive has then sold those products to its network of various stem cell clinics across the U.S. This distribution network is wholly different than the laboratories and healthcare workers it would need in order to successfully distribute a COVID-19 test.
We asked investor relations about this need for an entirely different distribution network in order to successfully provide this test. Their answer, once again, has been silence.
7. Where are the basic details? Beyond the above, we asked Predictive’s investor relations for basic details on its partnership with Dablood. We asked if there was a signed agreement, what the terms of such an agreement were, how many tests had been manufactured to date and whether any had been delivered to Predictive.
Once again, our questions have been met with total silence.
We Wrote Extensively About Predictive Last Summer. We Are Updating Our Conclusion From 95%+ Downside to Near-Term 100% Downside
We wrote extensively about Predictive in July 2019, when the stock was trading at a market cap of around $1 billion.
At the time, we called Predictive “a company displaying hallmarks of insider self-dealing that also seems to be taking advantage of vulnerable pregnant women and elderly patients suffering from chronic pain”.
With the stock at $3.40, we said we thought the stock could have 95%+ downside ($0.17 or less). We now think the company has near-term 100% downside. Predictive continues to strike us as a terrible investment opportunity given the slew major red flags and signs of insider self-dealing we’ve uncovered – the latest red flag being the company’s convenient pivot into COVID-19 testing during the height of the global pandemic.
We believe that should the company narrowly avoid insolvency, it will do so by continuing to dilute its equity, which will continue to put downward pressure on the company’s stock. The dilution risk, combined with a rapidly declining stem cell business (that the FDA has been more aggressively curtailing), continues to make Predictive an “avoid at all costs” type of investment.
Disclosure: We are short shares of Predictive (PINK:PRED)
Legal Disclaimer
Use of Hindenburg Research’s research is at your own risk. In no event should Hindenburg Research or any affiliated party be liable for any direct or indirect trading losses caused by any information in this report. You further agree to do your own research and due diligence, consult your own financial, legal, and tax advisors before making any investment decision with respect to transacting in any securities covered herein. You should assume that as of the publication date of any short-biased report or letter, Hindenburg Research (possibly along with or through our members, partners, affiliates, employees, and/or consultants) along with our clients and/or investors has a short position in all stocks (and/or options of the stock) covered herein, and therefore stands to realize significant gains in the event that the price of any stock covered herein declines. Following publication of any report or letter, we intend to continue transacting in the securities covered herein, and we may be long, short, or neutral at any time hereafter regardless of our initial recommendation, conclusions, or opinions. This is not an offer to sell or a solicitation of an offer to buy any security, nor shall any security be offered or sold to any person, in any jurisdiction in which such offer would be unlawful under the securities laws of such jurisdiction. Hindenburg Research is not registered as an investment advisor in the United States or have similar registration in any other jurisdiction. To the best of our ability and belief, all information contained herein is accurate and reliable, and has been obtained from public sources we believe to be accurate and reliable, and who are not insiders or connected persons of the stock covered herein or who may otherwise owe any fiduciary duty or duty of confidentiality to the issuer. However, such information is presented “as is,” without warranty of any kind – whether express or implied. Hindenburg Research makes no representation, express or implied, as to the accuracy, timeliness, or completeness of any such information or with regard to the results to be obtained from its use. All expressions of opinion are subject to change without notice, and Hindenburg Research does not undertake to update or supplement this report or any of the information contained herein.
23 thoughts on “Predictive’s COVID-19 Test Announcement Looks Like A Last-Ditch Sham To Salvage A Company On The Brink Of Insolvency”
Comments are closed.
Your joking right?
The article is a complete misrepresentation of what PRED has done with the test and the their partners in the test.
DaAn Gene is a $14 billion dollar publicly traded Chinese company – did you look them up.
These guys are shorts that work with Nate Anderson. He is being sued which it seems he likes from one of the Founders of PRED
Fucking lying POS outfit! Not only are you fucking investors but you are putting lives at stake
by disrupting testing. Do not show your face as have contracted Ray Donovan to go mid-evil!!!
Seriously the Russian Mafia will be paying you all a visit…
Yes very much so. PRED will have the last laugh
Haters gonna hate.